Q 10 Calculate the efficiency of packing in case of a metal crystal for
(i) simple cubic
(ii) body–centred cubic
(iii) face–centred cubic (with the assumptions that atoms are touching each other).
(i)
In a simple cubic lattice the atoms are located only on the corners of the cube.
Let take edge length or side of the cube = a,
The relation between radius and edge a
a = 2r
The volume of the cubic unit cell = side3
= a3
= (2r)3
= 8r3
Number of atoms in unit cell = 8 x 1 /8
= 1
The volume of the occupied space = (4/3)πr3
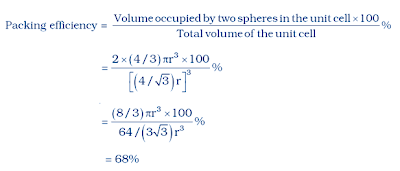
(ii) In body centered cubic two atoms diagonally
Let take edge length or side of the cube = a,
Let take radius of each particles = r
The diagonal of a cube is always a√3
The relation between radius and edge a will
a√3 = 4r
divide by root 3 we get
a = 4r/√3
total number of atoms in body centered cubic
number of atoms at the corner = 8 x 1/8 = 1
number of atoms at the center = 1
total number of atoms = 2
The volume of the cubic unit cell = side3
= a3
= (4r/√3)3
The volume of the occupied space = (4/3)πr3
.
(iii)
Let take edge length or side of the cube = a,
Let take radius of each particles = r
The diagonal of a square is always a√2
a√2 = 4r
divide by root 3 we get
a = 4r/√2
total number of atoms in body centered cubic
number of atoms at the corner = 8 x 1/8 = 1
number of atoms at the face = 6 x1/2 = 3
total number of atoms = 4
The volume of the cubic unit cell = side3
= a3
= (4r/√2)3
= (2√2 r)3
The volume of the occupied space = (4/3)πr3
.





No comments:
Post a Comment